
Collaboration partners
Uppsala University, Karolinska Institutet, Linköping University, Olink Proteomics
Welcome to DoloRadix AB
DoloRadix AB is a pain management company with a clinical focus, with the desire and curiosity to cure pain for patients all over the world.
Our misson is to radically change the daily management of musculoskeletal pain across all patient groups.
We engage with leading universities to develop new clinical concepts, including diagnostic tools and tools for decision support and treatments, that will profoundly change the recovery rate for patients with musculoskeletal pain, including a remarkable shortening of the treatment time, increase the quality of life for the individual patient, and lower the health care costs related to pain disorders.
Doloradix AB was established 2016 with the purpose to fully leverage the 20 years of experience from Mårten Prosell and his clinical work through Progress Ortopedisk Medicin AB. Doloradix AB will continue to explore and develop the unique documented clinical findings through development programs to allow for expanded access to its already successful methodologies, for the benefit of patients all around the world.
Publications
A qualitative study in Swedish track and field
Richard Thompson a*, Mårten Prosell b*, Toomas Timpkaa*
a, Athletics Research Center. Linköping University, Sweden
b, Doloradix AB, Sweden
Elite athletes’ experiences of musculoskeletal pain management using neuroanatomical dry needling (naDN): A qualitative study in Swedish track and field
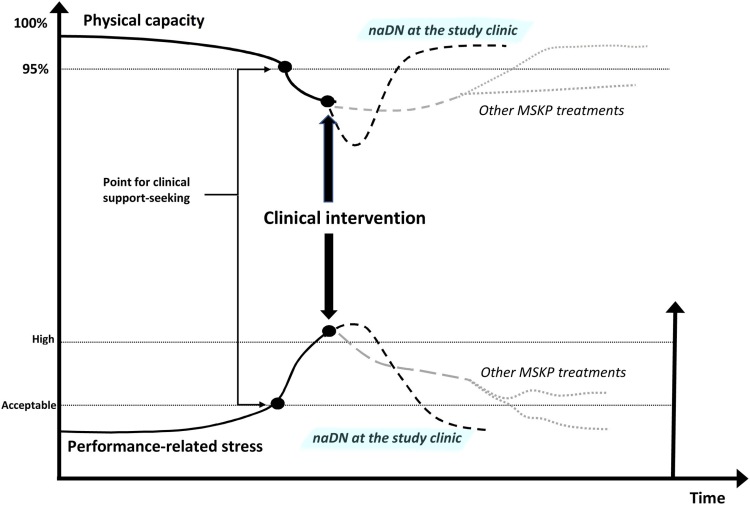
This qualitative study was based on the thematic analysis method.13 It is reported in line with the Consolidated Criteria for Reporting Qualitative Research (COREQ) guidelines.14 The study design was approved by the regional ethics committee in Linköping, Sweden (2018/484-31). All participation was voluntary and informed written consent was obtained from all participants. Participants were not involved in the design, conduct, reporting, or dissemination of this research.
The study was performed in the Swedish elite track and field setting. The inclusion criteria were (1) current or former elite athlete, (2) history of longstanding MSKP (>3 months), and (3) treated at the study clinic (2016–2018). Elite athlete was defined as a track and field athlete competing/having competed in senior championships at national or international level, e.g. the Swedish, European or World Championships. The study clinic specialises in treating MSKP through the application of naDN.
“You won’t become a great clinician behind the computer.”
– Mårten Prosell
News
Utforskning av det kliniska handhavandet av patienter med muskuloskeletal smärta i primärvården
Bakgrund: Smärta är vanligt förekommande och medför negativa konsekvenser för såväl individ somsamhälle. Vården av patienter med smärta tillhandahålls oftast av allmänläkare men brister i dess kvalitéhar beskrivits och forskning inom området är knapphändig. En klinik specialiserad på behandlingen avpatienter med muskuloskeletal smärta har beskrivits som framgångsrik i behandlingen av dessapatienter. Syfte: Att beskriva allmänläkares…
Read more…Mikrosensor för lokalisering av smärtkällor bland finansierade samverkansprojekt i utlysning från innovationsprogram
15 projekt som verkar för en bättre hälsa får dela på 36 miljoner kronor i innovationsprogrammen Swelifes och Medtech4Healths gemensamma utlysning. Ken Welch, professor vid institutionen för materialvetenskap vid Uppsala universitet, leder ett av de utvalda projekten. Utlysningen lockade inte mindre än 130 samverkansprojekt från hälso- och sjukvård, akademi/institut och industri att ansöka. Ett av…
Read more…
